
At the Maximum Recommended Dose, XYWAV Contains 1509 mg/Day Less Sodium Than All High Sodium Oxybates1-6
High-sodium oxybates, including XYREM and LUMRYZTM (sodium oxybate) for extended-release oral suspension, have ~1640 mg of sodium in a 9-g dose,3-6 which exceeds the American Heart Association’s ideal daily limit of 1500 mg/day for most adults.7,8
The difference in sodium content between a 9-g dose of high-sodium oxybates and XYWAV is nearly the sodium equivalent of 4 orders of large fries2-4,9,*
The difference in sodium content between a 9-g dose of high-sodium oxybates and XYWAV is nearly the sodium equivalent of 4 orders of large fries2-4,9,*
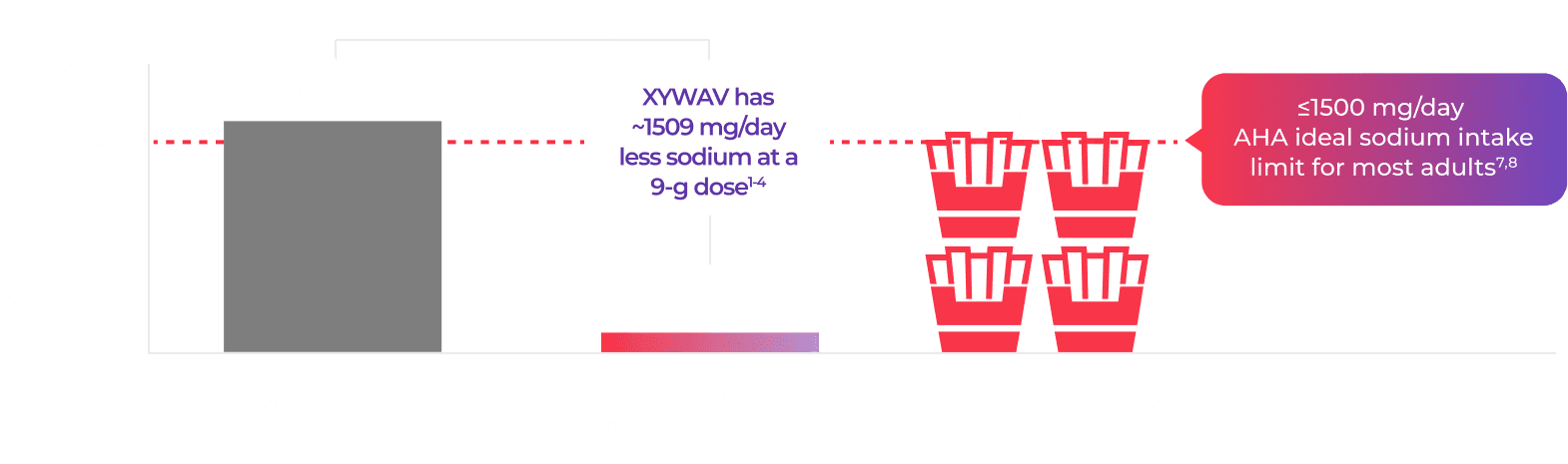
At the maximum recommended 9-g dose, there is a difference of 1509 mg in sodium between XYWAV and high-sodium oxybates like XYREM and LUMRYZ.1‑3,7,10‑12
High-Sodium Oxybates, Including XYREM and LUMRYZTM (Sodium Oxybate) for Extended-Release Oral Suspension, Have ~1640 mg of Sodium in a 9-g Dose3,4
There are no head-to-head data for XYWAV and XYREM or LUMRYZ.
XYWAV has 131 mg of sodium per maximum recommended 9-g nightly dose, while the equivalent dose of high-sodium oxybates XYREM® (sodium oxybate) oral solution and LUMRYZ™ (sodium oxybate) extended-release for oral suspension contain ~1640 mg—a difference of ~1509 mg every single day.2,4,10
Prescribing XYWAV instead of high-sodium oxybates like LUMRYZ or XYREM will reduce your patients’ sodium burden—and potentially, their cardiovascular risk.1‑4,10‑14
XYWAV Is the First and Only Low-Sodium Oxybate for the Treatment of Cataplexy and EDS in Patients With Narcolepsy Ages 7 and Older1,2,15,16,†

XYWAV Is Clinically Superior to the High-Sodium Oxybate XYREM on the Basis of Safety
Significantly reduce your patients’ chronic sodium burden by choosing low-sodium XYWAV, the only oxybate granted clinical superiority to XYREM on the basis of safety.2-4,13,14,19
§Clinically superior | According to the US Food and Drug Administration (FDA), XYWAV is clinically superior to XYREM® (sodium oxybate) oral solution due to greater cardiovascular (CV) safety because the reduction in sodium at recommended doses will help a substantial portion of indicated patients reduce the risk of developing CV disease. Based on the determination of orphan drug exclusivity by the FDA Office of Orphan Products Development (OOPD) between XYWAV and XYREM.13,19 There are no head-to-head data for XYWAV and XYREM.
AHA, American Heart Association; EDS, excessive daytime sleepiness; HHS, US Department of Health and Human Services; USDA, United States Department of Agriculture.
*Based on an average of 380 mg of sodium in 1 large serving of french fries according to a 2012 USDA analysis of 3 fast-food chains.9
†XYWAV contains 131 mg of sodium at the maximum recommended nightly dose.2
*Based on an average of 380 mg of sodium in 1 large serving of french fries according to a 2012 USDA analysis of 3 fast-food chains.9
†XYWAV contains 131 mg of sodium at the maximum recommended nightly dose.2
Important Safety Information
Warnings and Precautions (cont'd)
Other Behavioral or Psychiatric Adverse Reactions
In Study 1, the pivotal clinical trial in adults with narcolepsy, confusion and anxiety occurred in 1% and 5% of patients treated with XYWAV, respectively. One patient experienced visual hallucinations and confusion after ingesting approximately 9 grams of XYWAV.
Other neuropsychiatric reactions reported with oxybate (same active moiety as XYWAV) in adult or pediatric clinical trials and in the postmarketing setting include hallucinations, paranoia, psychosis, aggression, agitation, confusion, and anxiety. The emergence or increase in the occurrence of behavioral or psychiatric events in patients taking XYWAV should be carefully monitored.
Other neuropsychiatric reactions reported with oxybate (same active moiety as XYWAV) in adult or pediatric clinical trials and in the postmarketing setting include hallucinations, paranoia, psychosis, aggression, agitation, confusion, and anxiety. The emergence or increase in the occurrence of behavioral or psychiatric events in patients taking XYWAV should be carefully monitored.
Parasomnias
Parasomnias can occur in patients taking XYWAV.
In Study 1, parasomnias, including sleepwalking were reported in 6% of adult patients treated with XYWAV.
In a clinical trial of XYREM (same active moiety as XYWAV) in adult patients with narcolepsy, five instances of sleepwalking with potential injury or significant injury were reported. Parasomnias, including sleepwalking, have been reported in a pediatric clinical trial with sodium oxybate (same active moiety as XYWAV) and in postmarketing experience with sodium oxybate.
Episodes of sleepwalking should be fully evaluated, and appropriate interventions considered.
In Study 1, parasomnias, including sleepwalking were reported in 6% of adult patients treated with XYWAV.
In a clinical trial of XYREM (same active moiety as XYWAV) in adult patients with narcolepsy, five instances of sleepwalking with potential injury or significant injury were reported. Parasomnias, including sleepwalking, have been reported in a pediatric clinical trial with sodium oxybate (same active moiety as XYWAV) and in postmarketing experience with sodium oxybate.
Episodes of sleepwalking should be fully evaluated, and appropriate interventions considered.
Most Common Adverse Reactions
In Study 1, the most common adverse reactions (incidence ≥5% of XYWAV-treated patients with narcolepsy) were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis, anxiety, and vomiting.
In the pediatric clinical trial with XYREM (same active moiety as XYWAV), that included pediatric patients 7 to 17 years of age with narcolepsy, the most common adverse reactions (≥5%) were nausea (20%), enuresis (19%), vomiting (18%), headache (17%), weight decreased (13%), decreased appetite (9%), dizziness (8%), and sleepwalking (6%). The overall adverse reaction profile of XYREM in the pediatric clinical trial was similar to that seen in the adult clinical trial program. The safety profile in pediatric patients with XYWAV is expected to be similar to that of adult patients treated with XYWAV and to that of pediatric patients treated with XYREM.
In the pediatric clinical trial with XYREM (same active moiety as XYWAV), that included pediatric patients 7 to 17 years of age with narcolepsy, the most common adverse reactions (≥5%) were nausea (20%), enuresis (19%), vomiting (18%), headache (17%), weight decreased (13%), decreased appetite (9%), dizziness (8%), and sleepwalking (6%). The overall adverse reaction profile of XYREM in the pediatric clinical trial was similar to that seen in the adult clinical trial program. The safety profile in pediatric patients with XYWAV is expected to be similar to that of adult patients treated with XYWAV and to that of pediatric patients treated with XYREM.
References: 1. XYWAV® (calcium, magnesium, potassium, and sodium oxybates). Prescribing information. Palo Alto, CA: Jazz Pharmaceuticals, Inc. 2. Chen C et al. Clin Transl Sci. 2021;14(6):2278-2287. 3. XYREM® (sodium oxybate). Prescribing information. Palo Alto, CA: Jazz Pharmaceuticals, Inc. 4. LUMRYZ® (sodium oxybate). Prescribing Information. Dublin, Ireland: Avadel Pharmaceuticals, Inc; 2023. 5. Sodium oxybate oral solution. Prescribing information. Berkeley Heights, NJ: Hikma Pharmaceuticals USA Inc; 2023. 6. Sodium oxybate oral solution. Prescribing information. Bridgewater, NJ: Amneal Pharmaceuticals LLC; 2023. 7. American Heart Association. https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/sodium/how-much-sodium-should-i-eat-per-day. Accessed April 4, 2024. 8. Benjamin EJ et al. Circulation. 2019;139(10):e56-e528. 9. US Department of Agriculture, Agricultural Research Service. FoodData Central, 2019. fdc.nal.usda.gov. Accessed April 4, 2024. 10. Arnett DK et al. Circulation. 2019;104:e563-e595. 11. Whelton PK et al. Hypertension. 2018;71:e13-e115. 12. Ma Y et al. N Engl J Med. 2022;386(3):252-263. 13. US Food & Drug Administration. https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/clinical-superiority-findings. Accessed March 4, 2024. 14. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium, Stallings VA, Harrison M, Oria M, eds. Dietary Reference Intakes for Sodium and Potassium. Washington (DC): National Academies Press (US); 2019. 15. Voss SR, Kehoe TE. Center for Drug Evaluation and Research. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202344Orig1s000MedRv.pdf. Accessed February 28, 2023. 2011. 16. US Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/quantitative-labeling-sodium-potassium-and-phosphorus-human-over-the-counter-and-prescription-drug. Published September 2022. Accessed February 28, 2023. 17. Junnarkar G et al. Expert Opin Drug Discov. 2022;17(2):109-119. 18. Data on file. (JSP258-2020-013). Jazz Pharmaceuticals, Inc. 19. Data on file (XYW-2023-030). Jazz Pharmaceuticals, Inc.
Important Safety Information
WARNING: CENTRAL NERVOUS SYSTEM DEPRESSION and ABUSE AND MISUSE.
- Central Nervous System Depression
XYWAV is a CNS depressant. Clinically significant respiratory depression and obtundation may occur in patients treated with XYWAV at recommended doses. Many patients who received XYWAV during clinical trials in narcolepsy were receiving CNS stimulants.

